L’A83 CBE dispose :
L’invention doit être exposée dans la demande de brevet européen de façon suffisamment claire et complète pour qu’un homme du métier puisse l’exécuter.
Suffisance de description
Date d’appréciation
La suffisance de description, pour apprécier la validité d’un brevet / d’une demande, s’apprécie à la date de dépôt de la demande (Directives F-II 4.1).
Pour savoir si une divulgation anticipe une invention, l’appréciation de la suffisance de description est différente : c’est à la date de publication de cette divulgation qu’il faut se placer (ou de dépôt pour un document A54(3) CBE) (T206/83, T26/85 et Directives G-VI 4).
Critère d’appréciation
La suffisance de description s’apprécie par rapport (Directives F-II 4.1) :
- aux connaissances générales de base de l’homme du métier ;
- à l’invention décrite dans la demande ;
- les documents cités.
Si des produits sont revendiqués, seule leur fabrication doit être suffisamment décrite (T866/00) : il n’est pas utile de décrire leur utilisation.
Tests de vérification de la suffisance de description
Afin de vérifier que la description est suffisance pour la mise en œuvre de l’invention, les chambres de recours ont proposé un test en 4 étapes (T593/09, T2403/11) :
- Identification du problème résolu par l’invention ;
- La caractéristique arguée comme non suffisamment décrite (ex. seuil de porosité d’un matériau) est-elle pertinente pour résoudre ce problème ?
- Existe-t-il une ambiguïté possible quant à la mesure / estimation de cette caractéristique ?
- ex. plusieurs méthodes de mesures donnent des résultats différents, selon les conditions les mesures sont différentes (T2096/12) ;
- une mesure via une machine spécifique est mentionnée dans la description mais cette machine ne se fabrique plus (T1293/13) et il est impossible de savoir si les nouvelles machines donnent le même résultat ;
- L’ambiguïté est-elle telle que l’invention n’est pas suffisamment décrite ? (ex. discrimination des matériaux satisfaisants le problème technique n’est pas possible).
Une fois ce test déroulé, il devient possible de déterminer si l’invention est insuffisamment décrite.
Cas de la mesure des paramètres
Il convient de noter que des imprécisions quant à la mesure d’un paramètre ou l’existence de plusieurs méthodes de mesure ne conduisent pas nécessairement à une insuffisance de description (T608/07, T1768/15) mais souvent à des problèmes de clarté.
Néanmoins, dans un cas où le paramètre est crucial pour résoudre le problème sous-jacent à l’invention, la méthode de mesure doit donner des valeurs cohérentes de manière à ce que l’homme du métier sache, lorsqu’il reproduit l’invention, si ce qu’il produit résout le problème ou pas (T815/07, T1305/15) : c’est alors un problème de suffisance de description.
Cas des réseaux de neurones
Dans le cas d’invention utilisant un réseau de neurones, nous pouvons nous demander s’il est utile / nécessaire de décrire comment le réseau de neurones a été entrainé.
Cela est d’autant plus pertinent que l’entrainement des réseaux nécessitent plusieurs méga-octets (voir des gigas) et qu’il n’est pas vraiment envisageable d’inclure ses données dans le texte de la demande.
Selon la jurisprudence (T161/18), le simple fait d’indiquer de manière générale que les données d’apprentissage doivent couvrir un large périmètre (ex. âge de patient, sexe, etc.) n’est pas suffisant.
Il est probable qu’il faille indiquer précisément quels types de données et quels paramètres / hyper-paramètres sont utilisés. Mais dans quelles mesures, c’est une vrai question …
Cas des médicaments / composition pharmaceutique
Il peut arriver qu’une invention vise un médicament ou une composition pharmaceutique sans que la demande comporte de tests cliniques apportant la preuve que ce médicament / composition pharmaceutique ait l’efficacité / l’effet revendiqué.
Néanmoins, cela n’est pas suffisant pour qu’une objection de suffisance de description soit soulevée (T2015/20) : Une invention n’est pas suffisamment décrite que si elle va à l’encontre d’une opinion technique dominante et que le brevet ne fournit aucun exemple reproductible.
Ce n’est donc pas une question de plausibilité (qui relève alors de l’activité inventive).
Modes de réalisation décrits
Revendication plus large que les modes décrits
Principe
Selon certaines décisions, l’exposé doit être suffisant pour permettre de mettre en œuvre presque tous les modes couverts par la revendication (T409/91).
Néanmoins, d’autres décisions estiment que le critère de la suffisance de la description est rempli si au moins un mode de réalisation est décrit (T292/85 ou T389/94).
Il convient donc de regarder les faits : si l’invention est très novatrice, elle peut souvent être facilement généralisée une fois qu’on a guidé l’homme du métier vers un mode de réalisation (Directives F-III 1).
Ainsi, on acceptera plus facilement la description d’un seul mode de réalisation si l’invention est novatrice (Directives F-III 1).
Il convient d’admettre les tâtonnements dans une mesure limitée, par exemple lorsqu’il s’agit d’un domaine encore inexploré ou lorsque de nombreuses difficultés techniques se présentent (T292/85, T409/91 et Directives F-III 1)..
Une revendication large peut être admissible s’il est possible d’étendre les exemples donnés par des méthodes d’expérimentation ou d’analyse habituelles (Directives F-IV 6.3).
« Domaine interdit » d’une revendication ou objection de clarté maquillée
Il peut arriver qu’une caractéristique soit si peu claire que l’homme du métier soit dans l’impossibilité de savoir s’il travaille ou non dans le domaine revendiqué (« domaine interdit »).
De nombreux opposants ont souvent tenté d’argumenter que cela relevait d’un problème de suffisance de description (la clarté n’étant pas un motif d’opposition).
Mais aujourd’hui, les chambres de recours sont assez unanimes : la définition du domaine revendiqué était une question d’A84 CBE plus que d’A83 CBE (T1811/13).
Il convient de veiller à ce qu’une objection d’insuffisance de description naissant d’une ambiguïté ne soit pas une simple objection de clarté maquillée (T608/07, 2.5.2).
Mode de réalisation ou domaine de fonctionnement…
… ne fonctionnant pas dans toute la portée de la revendication
Charge de la preuve
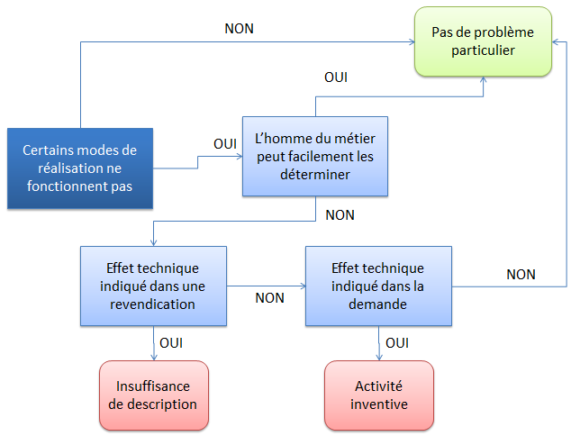
S’il est soutenu que, si l’on se conforme aux détails donnés par la description, certains domaines ou certains modes de réalisation ne fonctionnent pas, la partie soulevant ce problème doit fournir les éléments soutenant sa position (i.e. sérieuses réserves, étayées par des faits vérifiables, T409/91, T1057/22 et T694/92).
Pour autant, cela ne signifie pas que, dès qu’un domaine couvert par la revendication ne fonctionne pas, il existe une insuffisance de description. En effet, lorsqu’il est clair pour l’homme du métier qu’une telle domaine n’est pas raisonnable ou ne fonctionne pas (n’atteint pas l’effet technique), il n’y a pas de problème (T1943/15, T2773/18).
Pour autant, et toute la difficulté est vraiment là, une caractéristique limitative qui ne figure que dans la description ne peut être considérée comme implicite dans les revendications (T989/16).
Objection de suffisance de description
Si une revendication couvre plusieurs modes et que certains ne fonctionnent pas, la revendication devra être considérée comme insuffisamment décrite même si les autres modes fonctionnent correctement (T1173/00, T239/13).
Alternative à cette objection
Une objection relative à l’absence de caractère industriel (A52(1) CBE) peut également être soulevée (Directives F-III 3).
… ne fonctionnant plus
Il peut arriver qu’un mode de réalisation ne puisse plus être mis en œuvre.
Cela arrive lorsque ce dernier est tellement précis, qu’il faut une machine particulière pour le mettre en œuvre et que cette machine n’existe plus (ex. énergie d’un choc mesuré en utilisant l’appareil Drop-Weight Tester RTD-5000 de Rheometrics, Inc., T1714/15).
Dans ce cas, nous avons un problème de suffisance de description.
… ne fonctionnant pas toujours (lié au hasard)
Il peut arriver que la bonne exécution de l’invention dépende du hasard (Directives F-III 3).
Ainsi, les détails donnés dans la description ne permettent pas, de manière systématique, d’obtenir le résultat souhaité (Directives F-III 3) :
- fabrication de puces électronique ;
- procédé microbiologique impliquant des mutations ;
- etc.
Ces inventions ne seront considérées comme suffisamment décrite que si (Directives F-III 3) :
- des résultats satisfaisants peuvent être obtenus de manière répétée, même s’il y a des échecs ; et
- s’il est possible de savoir si le résultat est satisfaisant en utilisant des méthodes d’essai non destructif.
… ne fonctionnant pas toujours (fiabilité inférieure à 100%)
Il peut arriver que certains appareils mécaniques n’ait pas une fiabilité absolue (ex. appareil de tri mécanique).
Dès lors, l’homme du métier doit s’efforcer de faire preuve d’un esprit constructif et non destructeur en vue de parvenir à une interprétation de la revendication qui ait un sens du point de vue technique et tienne compte de l’ensemble de l’exposé de l’invention contenu dans le brevet (T383/14).
Ainsi, même si une machine de tri mécanique, occasionnellement, fait des erreurs de tri, l’invention sera considérée comme suffisamment décrite.
… difficiles à réaliser ou imparfaits
La difficulté de réalisation d’une invention ne signifie pas nécessairement que l’invention est insuffisamment décrite (Directives F-III 5.3).
Ainsi, un mode de réalisation ne fonctionnant pas parfaitement (ex. interrupteur électrique laissant passer, ouvert, un léger courant résiduel) et rendant certaines applications de l’invention inopérante que dans certains cas (ex. circuit électrique nécessitant un courant absolument nul lors de l’ouverture de l’interrupteur) sera considéré comme suffisamment décrit (Directives F-III 5.3).
… ne permettant pas de résoudre le problème technique
Si l’invention ne permet pas d’obtenir l’effet technique allégué, il n’est possible de rejeter la demande au titre de l’insuffisance de description que si cet effet technique est revendiqué (G1/03, point 2.5.2 ou T2001/12).
Néanmoins, si le fait de ne pas atteindre l’effet technique est lié (T2001/12) :
- à l’absence d’une caractéristique essentielle, une objection de clarté pourra être soulevée ;
- à l’impossibilité technique d’arriver à l’effet au regard des affirmations de l’art antérieur, une objection d’activité inventive pourra être soulevée.
Nous pouvons synthétiser comme suit la décision G1/03, point 2.5.2 :
… citant marques, noms propres, noms commerciaux
En effet, ces termes constituent simplement une indication d’origine et peuvent se rapporter à toute une gamme de produits différents.
Pour être suffisamment décrit, le produit doit être suffisamment identifié, sans référence à une quelconque marque, nom propre ou nom commercial, pour permettre la réalisation de l’invention, à moins que ces termes aient un sens précis pour l’homme du métier (par exemple câble « Bowden » , rondelle « Belleville » , barre « Panhard » , chenille « Caterpillar« ) (Directives F-III 7).
Si un produit d’une marque est mentionné mais que la composition et la méthode de fabrication dudit produit est secret, il y a fort à parier que l’exigence de suffisance de description n’est pas remplie (T797/14).
Nombre de modes de réalisations décrits
La description doit comporter, a minima, la description d’un mode de réalisation couvert par les revendications (Directives F-III 1), ce mode de réalisation fonctionnant (voir ci-dessus, Directives F-III 5.1).
Si un mode de réalisation peut suffire, il peut être judicieux d’en donner plusieurs notamment si la formulation des revendications est assez large et que les connaissances générales de l’homme du métier ne sont pas suffisantes pour extrapoler la réalisation des modes de réalisation non décrits, mais couverts (T727/95) par les revendications.
Méthode non « classique »
Lorsque le déposant choisit d’utiliser des méthodes non ordinaires (ex. de mesures), il convient de particulièrement les détailler afin de s’assurer que ces méthodes soient suffisamment décrites (T602/10).
Incorporation par référence
Une demande ne sera pas insuffisamment décrite si un élément essentiel de la description est mentionné par référence uniquement sans être repris in extenso.
La langue du document cité importe peu (T920/92).
La référence sera valable si (Directives H-V 2.5 ensemble Directives H-IV 2.3.1) :
- à la date de dépôt, une copie de ce document était à la disposition de l’OEB (ce qui est le cas si c’est une autre demande de brevet européen, T737/90) ;
- à la date de publication, ce document était accessible au public (T426/96).
Charge de la preuve
C’est d’abord à l’opposante/division d’Examen de démontrer, sur la base de la balance des probabilités, qu’un homme du métier serait incapable de reproduire l’invention, et si l’Opposante/division d’Examen s’est acquittée de sa charge de la preuve, cette dernière revient à la Titulaire qui cherche à réfuter les faits définitivement établis par des contre-arguments (Directives F-III 1, Directives F-III 4, T518/17).
Sanction
En cas d’insuffisance de description, il convient de limiter les revendications afin d’exclure les modes de réalisation insuffisamment décrits (Directives F-III 2).
De plus, il est impossible, après le dépôt, d’ajouter dans la demande la description manquante afin de « rendre » l’invention suffisamment décrite : en effet, une telle modification serait contraire à l’article A123(2) CBE.
Suffisance des revendications
Comme vous le savez tous, les revendications font parties de la description en Europe. Dès lors, ces dernières participent à la suffisance de la description (A83 CBE).
En vérité, il sera possible de soulever un problème de suffisance de description si les revendications ne présentent pas l’invention de manière suffisamment complète afin que l’invention puisse être réalisée (i.e. s’il manque par exemple des caractéristiques essentielle R43(1) CBE).
En effet, l’homme du métier doit trouver dans les revendications des indications sur les caractéristiques essentielles pour la mise en œuvre de l’invention dans toute sa portée. Et s’il ne le peut pas, nous avons un problème de suffisance (T623/16).
Règles de rédaction de la description
Principe
La R42 CBE définit précisément les règles auxquelles doit satisfaire la description.
Cette règle a pour objectif (Directives F-II 4.1)
- de garantir que la demande de brevet comporte des informations techniques suffisantes pour qu’un homme du métier puisse exécuter l’invention telle que revendiquée,
- de permettre à celui qui lit l’exposé de l’invention de comprendre la contribution apportée à l’état de la technique par l’invention telle que revendiquée.
État de la technique
Si le rapport de recherche fait apparaitre des documents pertinents, une référence à ces documents pourra être exigée (R42(1) b) CBE) ainsi qu’un bref résumé.
Cette introduction a posteriori ne contrevient pas à l’A123(2) CBE (T11/82).
Si l’Examinateur exige qu’une référence soit introduite, il faut respecter cette exigence sous peine de rejet de la demande (A97(2) CBE) ou de révocation du brevet (A101(3) b) CBE).
Si un art antérieur est A54(3) CBE, il est utile de le mentionner dans la demande (Directives F-II 4.3).
Problème technique et solutions
La R42(1) c) CBE demande qu’il soit possible de déduire le problème technique de la demande.
L’exposé du problème ne doit pas comprendre des déclarations dénigrantes (Directives F-II 4.5).
Il est possible de rédiger cette solution par référence aux revendications : « Ce problème est résolu par le dispositif de la revendication 1 » (cela n’est possible en France).
Description d’au moins un mode de réalisation
Il convient de regarder le chapitre sur la suffisance de description concernant cette description (R42(1) e) CBE).
Un simple copier-coller d’un programme informatique ne suffit pas à remplir cette condition (Directives F-II 4.12) même si cela est possible afin d’illustrer l’invention.
Application industrielle
Le plus souvent cette application industrielle (R42(1) f) CBE) se déduit directement de la demande et il n’est pas nécessaire de l’indiquer explicitement.
S’il n’est pas évident (ex. méthode d’essai), il faut l’expliciter (Directives F-II 4.9).
Sa mention est obligatoire pour une invention relative à une séquence de gènes (R29(3) CBE).
Incorporation par référence
Principe
Il est possible d’intégrer dans une revendication une caractéristique présente dans un document cité dans la demande, s’il est manifeste que ce document fait partie de l’invention pour laquelle une protection est recherchée (T6/84).
La langue de la citation importe peu (T920/92).
A123(2) CBE
Dès lors, si cette incorporation par référence est essentielle pour l’invention, le demandeur devra intégrer explicitement ces caractéristiques dans la description (Directives F-III 8).
Pour que cette intégration ne soit pas contraire à A123(2) CBE, il faut (Directives H-V 2.5, T689/90) :
- que la protection soit ou pourrait être recherchée pour ces caractéristiques ;
- que de telles caractéristiques concourent à la réalisation de l’objectif technique poursuivi par l’invention et font de ce fait partie de la solution du problème technique qui sous-tend l’invention revendiquée dans la demande ;
- que de telles caractéristiques sont de toute évidence comprises implicitement dans la description de l’invention que doit comporter la demande lors du dépôt (A78(1) b) CBE), et font donc partie du contenu de la demande telle qu’elle a été déposée (A123(2) CBE) ;
- que lesdites caractéristiques sont définies de façon précise et peuvent être identifiées parmi l’ensemble des informations techniques que renferme le document de référence.
La seule citation d’un document n’est a priori pas suffisante (T276/99). En revanche, l’indication « le polymère du document D1 est avantageux » est suffisamment précise.
Accessibilité au public de la référence
La référence sera valable si (Directives H-V 2.5 ensemble Directives H-IV 2.3.1) :
- à la date de dépôt, une copie de ce document était à la disposition de l’OEB (ce qui est le cas si c’est une autre demande de brevet européen, T737/90) ;
- à la date de publication, ce document était accessible au public (T426/96).
Éléments prohibés
Il ne faut pas que la description contienne (R48(1) CBE) :
- des éléments contraires à l’ordre public ;
- des déclarations dénigrantes ;
- des éléments étrangers ou superflus.
La section de dépôt vérifie ces conditions (A90(3) CBE) et omet ces éléments dans la publication de la demande (Directives A-III 8.1).
Si de tels éléments ne sont trouvés qu’au stade de l’examen, la division d’examen demandera leur suppression (Directives F-II 7.5).
Dépôt de matière biologique
Introduction
Ce thème est abordé ici, car, même si cela ne fait pas exactement partie de la description, le dépôt de matière biologique est un élément clef pour la suffisance de description.
Définition de matière biologique
On parle de matière biologique lorsque cette matière (R26(3) CBE) :
- contient des informations génétiques ;
- et est, dans un système biologique :
- autoreproductible ou
- reproductible.
Conditions à remplir
Principe
Afin de remplir à l’exigence de suffisance de description, il faut :
- qu’un échantillon de la matière biologique a été déposé auprès d’une autorité de dépôt habilitée (R31(1) a) CBE) :
- cf. Traité de Budapest du 28 avril 1977 ;
- au plus tard à la date de dépôt de la demande (pour permettre à l’Examinateur d’y avoir accès, Directives F-III 6.2) ;
- les autorités de dépôt habilitées (environ 40) sont mentionnées (« 4.2 Autorités de dépôt internationales selon l’article 7 du Traité de Budapest » , JO 2012, 324, II.1.4 et I.4.2) :
- que la demande contienne les informations pertinentes dont dispose le demandeur sur les caractéristiques de la matière biologique (R31(1) b) CBE). En général, ces informations pertinentes sont (Directives F-III 6.3) :
- la classification de la matière biologique (ex. : pour les bactéries, la littérature pertinente pour la classification sera R.E. Buchanan, N.E. Gibbons : Bergey’s Manual of Determinative Bacteriology) ;
- les différences importantes par rapport aux matières biologiques connues ;
- les caractéristiques morphologiques et biochimiques, dans la mesure où le déposant dispose de ces informations ;
- les informations utiles pour la reconnaissance et la reproduction ou la multiplication de la matière biologique, par exemple les milieux appropriés (composition d’ingrédients)) ;
- la description taxonomique proposée, dans la mesure où le déposant dispose de ces informations ;
- si la matière biologique déposée ne peut se reproduire elle-même, mais doit être reproduite dans un système biologique (p.ex. des virus ou de l’ARN libre), les informations susmentionnées pour ce système biologique (éventuellement associé à un dépôt de ce système biologique) ;
- que soit fourni, pour la matière biologique déposée :
- l’indication de l’autorité de dépôt (R31(1) c) CBE) ;
- l’indication du numéro d’ordre (R31(1) c) CBE).
- si la matière biologique a été déposée par une personne autre que le demandeur, que soit fournie (R31(1) d) CBE) ;
- le nom et l’adresse du déposant
- un document prouvant que le déposant
- a autorisé le demandeur à se référer dans la demande à la matière biologique déposée et
- a consenti sans réserve et de manière irrévocable à mettre la matière déposée à la disposition du public.
Pour autant, le simple dépôt d’une matière biologique ne peut constituer une demande de brevet en soi (T418/89).
Cas du renvoi à une demande antérieure
Lorsqu’une demande est déposée par renvoi à une demande antérieure, les conditions suivantes sont réputées être remplies à la date de dépôt dans la mesure ou celles-ci ont été remplies à la date de dépôt de la demande antérieure (Directives A-IV 4.1.2) :
- informations pertinentes dont dispose le demandeur sur les caractéristiques de la matière biologique (R31(1) b) CBE) ;
- indication de l’autorité de dépôt de la matière biologique déposée (R31(1) c) CBE) ;
- indication du numéro d’ordre de la matière biologique déposée (R31(1) c) CBE).
Procédure
Vérification
La section de dépôt vérifie, lors de l’examen quant à certaines irrégularités (A90(3) CBE), si la demande satisfait aux exigences de la R31(1) c) CBE et R31(1) d) CBE (Directives A-III 1.2 iv).
Si la section de dépôt constate que les informations sont manquantes, elle en informe le déposant (Directives A-IV 4.2).
Délai pour la correction
Le délai pour corriger cette irrégularité est de (R31(2) CBE, min des délais suivants) :
- 16 mois à compter de la priorité (ou tout du moins avant la fin des préparatif technique en vue de la publication) ;
- avant toute demande de publication anticipée selon A93(1) b) CBE ;
- 1 mois après la notification du demandeur qu’un tiers peut consulter le dossier, car une action a été intentée contre lui (A128(2) CBE).
L’A122 CBE est applicable.
Ce délai ne court donc pas à compter de la notification indiquant l’irrégularité.
Si jamais les informations de la R31(1) c) CBE et R31(1) d) CBE sont fournies après, la demande sera insuffisamment décrite (Directives F-III 6.3) quand bien même elles seraient fournies après :
- via une correction de la R56 CBE,
- via la fourniture d’une demande antérieure à laquelle il est fait renvoi selon la R40 CBE.
Rejet de la demande
Si une disposition de la R31(1) CBE n’est pas respectée, la suffisance de description n’est pas remplie et la demande est rejetée en vertu de l’A97(2) CBE (Directives F-III 6.3).
Dans ce cas, la perte de droit se produit dès le dépôt (même si une date de dépôt est attribuée).
Ainsi, même si la demande est rejetée, la priorité de cette première demande pourra être valablement revendiquée par une deuxième demande si et seulement si pour cette première demande, un échantillon a été déposé au plus tard à la date de dépôt de la première demande (i.e. R31(1) a) CBE était respecté) par application de A88(4) CBE (T193/95).
Accès à la matière biologique
Principe
Normalement, à compter de la publication de la demande de brevet, la matière biologique est accessible à toute personne qui le demande (ou avant par un tiers si une action est intentée contre ce tiers selon A128(2) CBE) (R33(1) CBE) :
- si le déposant l’a demandé avant la fin des préparatifs techniques en vue de la publication (R32(1) CBE) :
- un échantillon est remis à un expert si la demande d’accès est faite :
- avant la publication de la mention de la délivrance (R32(1) a) CBE) ;
- avant l’expiration d’un délai de 20 ans à compter du dépôt si la demande est rejetée / retirée / réputée retirée (R32(1) b) CBE) ;
- les experts peuvent être :
- toute personne physique désigné la personne souhaitant accéder à la matière biologique, s’il prouve lors de cette requête d’accès que le déposant est d’accord (R32(2) a) CBE) ;
- une personne agrée par le président de l’OEB (moins de 20 experts qui n’ont pas changé depuis 1992, liste dans « 1.5 Experts en microbiologie agréés par le Président de l’OEB conformé- ment à la règle 32, paragraphe 2, lettre b CBE » , JO 2012, 324 qui renvoie à « Liste des experts agréés aux fins de la R28 CBE » , JO 1992, 470) (R32(2) b) CBE) ;
- un échantillon est remis à un expert si la demande d’accès est faite :
- sinon,
- un échantillon est alors remis directement à cette personne.
Restriction quant à l’utilisation
Néanmoins, la personne qui a accès à cette matière doit s’engager à (R33(2) CBE) (jusqu’à ce que le brevet soit éteint dans tous les États ou jusqu’à ce que la demande soit rejetée / retirée / réputée retirée) :
- ne pas la communiquer à un tiers (même de façon dérivée) :
- sauf si le déposant l’y autorise,
- sauf si la matière qui en est dérivée est communiquée pour effectuer un dépôt de matière biologique (R33(3) CBE) pour une procédure de brevet ;
- à ne l’utiliser qu’à des fins expérimentales :
- sauf si le déposant l’y autorise ;
- sauf si cette personne possède une licence obligatoire.



Ping : Coronavirus : Le point sur le Brevet EP 1 694 829 B1 - Simple Curiosité