Pour être brevetable, une invention doit être nouvelle (A52 CBE).
Définition de la nouveauté
Une invention est nouvelle si elle n’est pas divulguée par l’état de la technique (A54(1) CBE).
Pour la définition de l’état de la technique, merci de se référer à La définition de l’état de la technique opposable.
La preuve d’une divulgation
Concernant la preuve d’une divulgation, merci de se référer à la page Preuves d’une divulgation.
Utilisation des divulgations pour attaquer la nouveauté
Principe : pas de combinaison
Pour la nouveauté, il faut normalement se limiter à un seul document et dans ce document à un seul mode de réalisation (T291/85 et Directives G-VI 1).
En effet, si on pouvait combiner toutes les divulgations dans un seul document (i.e. quelque soit le mode de réalisation), il serait facile de prendre un gros catalogue et de puiser les enseignements techniques souhaités un peu au hasard (T305/87, cisailles).
Exceptions
Cependant, un enseignement général d’un document peut être utilisé dans un exemple particulier de ce document (T1046/97) sauf si cet exemple est en contradiction avec le reste du document (T332/87).
Une combinaison est également possible si :
- cette combinaison ait été expressément suggérée (T305/87) (et que ceux-ci étaient accessible à la date de dépôt, Directives G-VI 8 et T153/85) ;
- cette combinaison ait pu être sérieusement envisagée par l’homme du métier (T666/89), par exemple si cela est suggéré de manière non ambigüe.
Le contenu qui pourra être combiné sera alors le contenu auquel il est fait référence : pas plus.
Bien entendu, il est possible d’utiliser des dictionnaires techniques ou des ouvrages de référence (même postérieurs à la demande) afin d’interpréter les revendications (Directives G-VI 1).
Évaluation de la nouveauté en fonction de la formulation des revendications à attaquer
Invention et plage de valeurs
Introduction
Une invention de sélection consiste à sélectionner (Directives G-VI 8), dans un ensemble connu ou une plage connue :
- des éléments individuels,
- des sous-ensembles,
- des sous-plages de valeurs limitées qui n’ont pas été mentionnés explicitement.
Cette sélection ne doit pas être le résultat obligatoire d’un procédé suffisamment décrit dans l’art antérieur (T12/81).
Sélection dans une liste individualisée
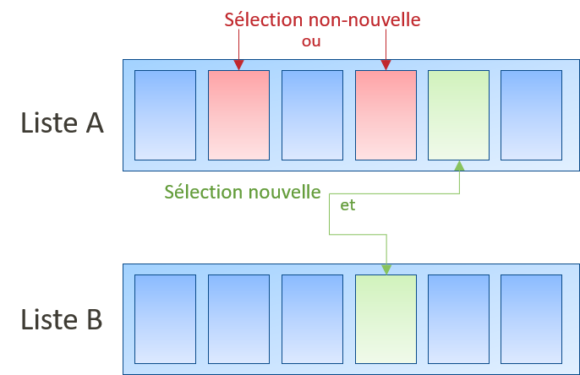
Une sélection n’est pas nouvelle si, pour la sélection dans une liste d’éléments individualisés, la sélection n’est effectuée que dans une unique liste (Directives G-VI 8 i, ex. sélection du caoutchouc dans une liste comprenant un ressort, du caoutchouc, etc.).
Néanmoins, si la sélection d’éléments est effectuée dans deux (ou plus) listes pour former une combinaison de caractéristiques (Directives G-VI 8 i, sélection du caoutchouc dans une liste comprenant un ressort, du caoutchouc, etc., et du fer dans une liste comprenant le cuivre, le fer, etc.).
On peut quand même s’interroger de la cohérence de cette approche avec la décision T1374/07 qui considère (pour des question de 123(2) qu’une une sélection de deux composés dans une liste unique équivaut à une sélection de composés dans deux listes identiques…
Sélection dans une liste individualisée courte
Néanmoins, si les listes sont courtes (i.e. quelques éléments), les jurisprudences précédentes ne s’appliquent pas car il sera beaucoup plus simple pour l’homme du métier de combiner (T2350/16).
Sélection d’une sous-plage dans une plage divulguée
Approche historique
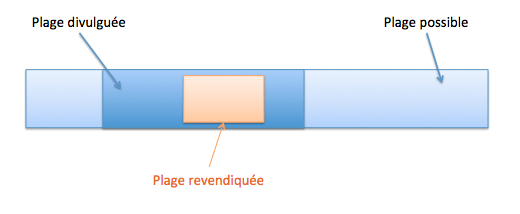
Une sélection peut être nouvelle si, pour la sélection d’une sous-plage dans une plage de valeurs connues (T261/15, T279/89, Directives G-VI 8 ii), cumulativement les conditions suivantes sont satisfaites :
- la sous-plage sélectionnée est étroite par rapport à la plage de valeurs connue ;
- la sous-plage sélectionnée est suffisamment éloignée de tout exemple spécifique divulgué dans l’état de la technique et des points extrêmes de la plage de valeurs connue (T17/85).
Il peut arriver que le document de l’art antérieur propose une composition de produits dont chaque produit est dans une plage donnée et que l’invention revendiquée propose une même liste de produits dans des sous-plages. Pour estimer si les sous plages sont étroites, il convient :
- de comparer individuellement chaque sous-plage par rapport à la plage correspondante, si la teneur des différents produits ne sont pas liées ;
- de comparer globalement les sous-plages par rapport aux plages divulguées, si la teneur des différents produits sont liées (T324/13).
Une sélection arbitraire ou sans effet technique ?
Pendant longtemps, l’OEB a considéré qu’il fallait en plus que la sélection ne soit pas arbitraire et soit réalisée avec un but particulier (i.e. en gros que la sélection ait un effet technique)
Cette ancienne approche n’est plus applicable car l’effet technique de la sélection n’est pertinente que pour analyser l’activité inventive (T1130/09, T1233/05, T230/07, T1948/10).
Sélection d’une sous-plage recoupant une plage divulguée
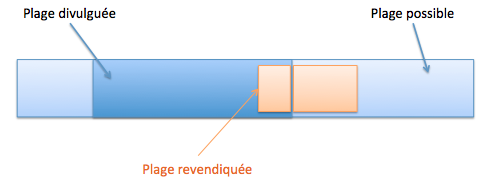
Une sélection peut être nouvelle si, pour la sélection d’une sous-plage recoupant partiellement une plage de valeurs connues (T666/89, Directives G-VI 8 ii), cumulativement :
- la sous-plage sélectionnée est suffisamment éloignée de tout exemple spécifique divulgué dans l’état de la technique et des points extrêmes de la plage de valeurs connue (T17/85).
- les valeurs divulguées (bornes ou valeurs intermédiaires divulguées) sont exclues par disclaimer,
- l’homme du métier n’aurait pas envisagé sérieusement de travailler dans la zone de recoupement, car l’état de la technique contenait une déclaration motivée le dissuadant clairement de sélectionner ladite plage de valeurs, bien que celle-ci ait été divulguée (ex. « il est possible de travailler dans la plage, [0-100°C] mais les basses températures donnent de mauvais résultats » T26/85).
La jurisprudence T26/85 s’applique par analogie pour liste d’éléments individualisés (T17/85).
Sélection d’une plage contenant une valeur divulguée
Comme le paragraphe précédent, il est nécessaire d’exclure la valeur divulguée afin que la revendication soit nouvelle.
Focus sur la valeur des bornes et valeurs divulguées
Lorsqu’une valeur numérique est donnée (individuellement ou dans une liste), il faut comprendre celle-ci comme une plage de valeur couvrant la marge d’erreur de la mesure (Directives G-VI 8.1).
Si aucune marge n’est indiquée, il faut regarder la plage de valeur permettant de faire l’arrondi (ex. [3,45cm ; 3,54cm] pour une indication de 3,5cm, Directives G-VI 8.1).
A titre d’illustration, si une revendication indique qu’un angle doit être strictement supérieur à 0°, nous comprenons sans difficulté qu’il peut être virtuellement impossible de faire la différence entre 0° et 0,000001°. Dès lors, la divulgation de 0° anticipe « un angle doit être strictement supérieur à 0° » (T386/17).
Invention d’utilisation
Un nouvel usage d’un produit connu est considéré comme nouveau pour le droit des brevets (i.e. « Usage du produit X pour… « ) même si cette nouvelle utilisation ne requiert pas de réalisation technique.
On parle bien de nouvel usage et non de nouvel effet technique : ainsi, si un nouvel effet technique est découvert, mais « explique » simplement un usage connu (ex. un effet désodorisant) la revendication ne sera pas nouvelle (T892/94 ou T2243/16).
Disclaimers
Disclaimer divulgué
Il est possible de prévoir dès le dépôt une caractéristique négative (Directives H-V 3.5) si :
- il n’existe pas de moyen plus clair et concis de protéger l’objet revendiqué, ou
- une caractéristique positive limiterait indûment la portée de la revendication.
Disclaimer non divulgué
Principe
Sous certaines conditions, il peut être possible de modifier spécifiquement ses revendications afin de regagner la nouveauté. Cette modification consiste en une formulation négative insérée dans les revendications afin d’exclure un art antérieur (Directives H-V 4.1).
Le disclaimer est accepté une des conditions suivantes est remplie (G1/03) :
- le disclaimer vise à rétablir la nouveauté en excluant une antériorité A54(3) CBE ;
- le disclaimer vise à rétablir la nouveauté en excluant une antériorité A54(2) CBE sachant que cette antériorité est fortuite (ex. problème technique totalement différent) ;
- le disclaimer vise à exclure un mode de réalisation exclu de la brevetabilité par A52 CBE à A57 CBE pour des raisons non techniques (ex. clonage humain).
Le disclaimer doit également respecter les conditions suivantes (G1/03) :
- le disclaimer doit être le plus restreint possible pour accomplir le but recherché sous peine d’être contraire à l’A123(2) CBE (sachant que par la suite la tenaille A123(2) CBE – A123(3) CBE s’appliquera, T747/00) à moins que cela soit obligatoire pour éviter un manque de clarté (T10/01) ;
- le disclaimer ne doit pas permettre d’échapper à un document au titre de l’activité inventive ;
- le disclaimer ne doit pas permettre d’échapper à une tenaille A123(2) CBE – A123(3) CBE (T1180/05) ;
- le disclaimer ne doit pas permettre d’écarter un mode de réalisation qui ne fonctionne pas ;
- le disclaimer ne doit pas permettre de pallier une insuffisance de description ;
- le disclaimer doit être clair et concis (T286/06).
Disclaimer par une caractéristique positive ?
Nous l’avons dit précédemment, un disclaimer doit être une caractéristique négative : il doit retrancher quelque chose de la revendication.
Ainsi, il n’est pas possible d’ajouter un disclaimer avec une caractéristique positive (T2502/13). Par exemple, si un art antérieur précise qu’un matériau n’est pas en plastique, il n’est pas possible d’indiquer que ce matériau est en plastique pour se distinguer de l’art antérieur.
Cela semble évident, mais cela va mieux en le disant 🙂
Catégorie de la revendication
Applications thérapeutiques
Première application thérapeutique
Ce que cela couvre
La première application thérapeutique est prévue à l’A54(4) CBE.
Cet article prévoit qu’il est possible de revendiquer un produit non nouveau pour une utilisation comme médicament : la revendication sera alors nouvelle (si bien sûr l’utilisation de ce produit comme médicament n’était pas connue).
La revendication sera alors formulée comme suit :
Produit X pour utilisation comme médicament, dans lequel…
La formulation de type « Utilisation de la substance X pour le traitement d’une maladie » (Directives G-VI 7.1) est interdite, car les méthodes thérapeutiques sont exclues de la brevetabilité (A53 c) CBE).
Ce que cela ne couvre pas
Pour autant, cette exception est limitée aux « substances ou compositions » : un simple dispositif thérapeutique (ex. un scalpel, une IRM, etc.) ne bénéficiera pas de cette exception (T2369/10).
Deuxième application thérapeutique
Ce que cela couvre
La deuxième application thérapeutique est prévue à l’A54(5) CBE.
Cet article prévoit qu’il est possible de revendiquer un produit (i.e substance ou composition (A54(5) CBE) actif (G5/83) non nouveau pour soigner :
- une maladie particulière ;
- une maladie qui se soigne habituellement avec ce produit, mais d’une nouvelle façon (G2/08, dosage différent).
La revendication sera alors nouvelle (si bien sûr l’utilisation de ce produit pour soigner cette maladie n’était pas connue).
La revendication sera alors formulée comme suit (Directives G-VI 7.1) :
Produit X pour utilisation dans le traitement de la maladie Y, dans lequel…
Nota : les revendications de type suisse (i.e « Utilisation d’une substance ou composition X pour l’obtention d’un médicament destiné à une utilisation thérapeutique Z » ou « Méthode pour fabriquer un médicament destiné à une utilisation thérapeutique Z, caractérisée en ce que la substance X est utilisée« ) ne sont plus admis pour les demandes ayant une la date de dépôt ou une date de priorité antérieure au 29 janvier 2011 (G2/08).
Ce que cela ne couvre pas
Néanmoins, il n’est pas possible d’utiliser les dispositions de l’article A54(5) CBE pour protéger un dispositif, quand bien même celui-ci serait un dispositif médical (ex. membrane de dialyse, T773/10 ou T2369/10).
Par ailleurs, l’utilisation d’une substance « non-active » (ex. un polymère permettant de donner une structure à une paroi du corps humain) n’est pas possible dans le cadre de l’A54(5) CBE (T2136/15).
Pour autant, une substance peut être inerte chimiquement et être active au sens de T2003/08 dès lors que cette substance a un effet thérapeutique (ex. produit un effet bénéfique sur le corps humain, T264/17).
Procédé
Il n’y a pas grand-chose à dire ici : toutes les caractéristiques du procédé doivent être reproduites.
Procédé pour + destination
Pour ce type de revendication, il convient de considérer cette « destination » comme étant une limitation de la revendication (T201/14 ou T1931/14).
La formulation « pour refondre des couches galvaniques » ne doit pas être comprise comme signifiant que le procédé est uniquement adapté à la refonte des couches galvaniques, mais comme une caractéristique fonctionnelle (G2/88) qui concerne la refonte des couches galvaniques et qui définit par conséquent l’une des étapes du procédé revendiqué (T848/93, Directives F-IV 4.13).
Néanmoins, il faut faire attention car un « procédé pour … » n’implique pas nécessairement la présence d’une caractéristique fonctionnelle mais peut seulement exprimer un but/effet à atteindre (T1338/18).
Il n’est pas nécessaire que cette destination soit suffisamment décrite pour devenir une caractéristique fonctionnelle (T1099/16).
Procédé pour + fabrication/obtention d’un produit
Contrairement à ce qui est mentionné pour les « procédés pour + destination » , le procédé qui conduit à l’obtention d’un produit doit être interprété en ce sens qu’il doit être simplement adapté à cette utilisation (T304/08).
Il convient de noter la décision T268/13 allant dans un sens contraire : selon elle, seuls les procédés de fabrication qui aboutissent à un tel produit peuvent être destructeurs de nouveauté.
Procédé pour + effet
Le fait d’ajouter un effet technique (qui est donc normalement inhérent aux étapes positives du procédé) n’est pas limitatif (T848/93 ou T1931/14).
Procédé de fabrication d’un produit nouveau et inventif
Lorsque la revendication de produit est brevetable, il n’est pas utile de regarder la nouveauté et le caractère inventif d’une revendication de procédé conduisant inévitablement à la fabrication de ce produit (T119/82, Directives F-IV 3.8 et Directives G-VII 13).
Produit
Il n’y a pas grand-chose à dire ici : toutes les caractéristiques du produit doivent être reproduites.
Normalement, une revendication de produit confère une protection absolue (dans n’importe quel contexte, G2/88)
Produit défini par un procédé (« product by process »)
La formulation de ces revendications est « Produit susceptible d’être obtenu par le procédé Y » ou équivalent (formulation à préférer par rapport à la formulation « Produit obtenu par le procédé Y » , T728/98).
En effet, ce produit ne doit pas être considéré comme nouveau du seul fait que le procédé de fabrication est nouveau : le produit en tant que tel doit satisfaire aux conditions de brevetabilité (Directives F-IV 4.12 et T150/82).
Dès lors, une telle revendication peut tout à fait être anticipée par un produit fabriqué par une autre méthode (pour autant qu’il n’existe pas de caractéristique intrinsèque du procédé de fabrication qui se retrouve dans le produit) : la charge de la preuve de la différence éventuelle repose néanmoins sur le demandeur (T205/83).
Ce type de revendication ne doit servir que s’il est impossible de définir le produit autrement.
Produit pour + destination
Normalement, une revendication de produit confère une protection absolue (dans n’importe quel contexte, G2/88).
Ainsi, une revendication « Produit pour + destination » devrait être vue comme équivalente à la revendication « Produit » (Directives G-VI 7) : une substance X qui est destinée à être utilisée comme catalyseur ne sera pas considérée comme nouvelle par rapport à la même substance connue comme colorant.
En revanche, si la destination de l’objet « cache » certaines caractéristiques techniques, cela sera différent : par exemple, « Dispositif pour mélanger du métal en fusion » suppose que ce dispositif supporte les grandes chaleurs.
En tout état de cause, pour analyser ce type de revendication, il convient de transformer les expressions « dispositif pour + destination » par « dispositif convenant à + destination » (Directives F-IV 4.13).
Utilisation
Les revendications d’utilisation doivent s’analyser comme des revendications de procédés (Directives F-IV 4.16).
Utilisation d’un produit nouveau et inventif
Lorsque la revendication de produit est brevetable, il n’est pas utile de regarder la nouveauté et le caractère inventif d’une revendication d’utilisation de ce produit (T642/94, Directives F-IV 3.8 et Directives G-VII 13).
Utilisation d’un procédé pour + destination
Ce type de revendication est équivalent à une revendication portant sur ce procédé (T684/02 et Directives F-IV 4.16).
Caractéristiques non techniques
Si une caractéristique est considéré comme non-technique, il convient de l’écarter pour l’analyse de la nouveauté (T619/98, G2/88, T959/98, T154/04).
Dans le cas d’une invention combinant des caractéristiques techniques et non techniques, on ne peut conclure que l’objet revendiqué n’est pas une invention parce que seules les caractéristiques non techniques apportent une contribution à l’état de la technique (T154/04).
Une caractéristique a un caractère non-technique notamment si l’effet de cette caractéristique est subjective (T1259/08). A titre personnel, je trouve cette approche étrange (tout du moins pour l’analyse de la nouveauté) car une caractéristique donnée pourrait être revêtue d’un caractère technique ou non en fonction de la formulation de son effet technique (ex. le zoom sur une image permet un effet graphique agréable ; le zoom sur une image permet de mieux distinguer les détails, etc.).
Exemples
Les expressions telles que « de préférence » , « par exemple » , « tels que » , etc. ne sont pas limitatifs et sont ignorées (Directives F-IV 4.9).
Explication d’un effet technique
Une nouvelle explication d’un effet technique ne confère pas de nouveauté (T892/94).